
John Rogers
Co-corresponding author
Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering and Neurological Surgery
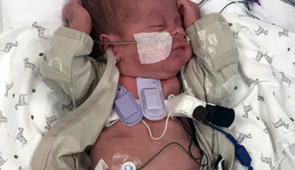
New devices were tested on a range of patients, from premature babies to the elderly
EVANSTON, Ill. — During even the most routine visits, physicians listen to sounds inside their patients’ bodies — air moving in and out of the lungs, heart beats and even digested food progressing through the long gastrointestinal tract. These sounds provide valuable information about a person’s health. And when these sounds subtly change or downright stop, it can signal a serious problem that warrants time-sensitive intervention.
Now, Northwestern University researchers are introducing new soft, miniaturized wearable devices that go well beyond episodic measurements obtained during occasional doctor exams. Softly adhered to the skin, the devices continuously track these subtle sounds simultaneously and wirelessly at multiple locations across nearly any region of the body.
The new study was published today (Nov. 16) in the journal Nature Medicine.
In pilot studies, researchers tested the devices on 15 premature babies with respiratory and intestinal motility disorders and 55 adults, including 20 with chronic lung diseases. Not only did the devices perform with clinical-grade accuracy, they also offered new functionalities that have not been developed nor introduced into research or clinical care.
“Currently, there are no existing methods for continuously monitoring and spatially mapping body sounds at home or in hospital settings,” said Northwestern’s John A. Rogers, a bioelectronics pioneer who led the device development. “Physicians have to put a conventional, or a digital, stethoscope on different parts of the chest and back to listen to the lungs in a point-by-point fashion. In close collaborations with our clinical teams, we set out to develop a new strategy for monitoring patients in real-time on a continuous basis and without encumbrances associated with rigid, wired, bulky technology.”
“The idea behind these devices is to provide highly accurate, continuous evaluation of patient health and then make clinical decisions in the clinics or when patients are admitted to the hospital or attached to ventilators,”said Dr. Ankit Bharat, a thoracic surgeon at Northwestern Medicine, who led the clinical research in the adult subjects. “A key advantage of this device is to be able to simultaneously listen and compare different regions of the lungs. Simply put, it’s like up to 13 highly trained doctors listening to different regions of the lungs simultaneously with their stethoscopes, and their minds are synced to create a continuous and a dynamic assessment of the lung health that is translated into a movie on a real-life computer screen.”
Rogers is the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering and Neurological Surgery at Northwestern’s McCormick School of Engineering and Northwestern University Feinberg School of Medicine. He also directs the Querrey Simpson Institute for Bioelectronics. Bharat is the chief of thoracic surgery and the Harold L. and Margaret N. Method Professor of Surgery at Feinberg. As the director of the Northwestern Medicine Canning Thoracic Institute, Bharat performed the first double-lung transplants on COVID-19 patients in the U.S. and started a first-of-its-kind lung transplant program for certain patients with stage 4 lung cancers.
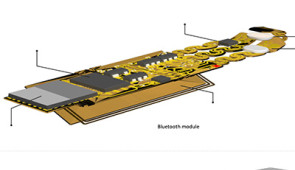
Containing pairs of high-performance, digital microphones and accelerometers, the small, lightweight devices gently adhere to the skin to create a comprehensive non-invasive sensing network. By simultaneously capturing sounds and correlating those sounds to body processes, the devices spatially map how air flows into, through and out of the lungs as well as how cardiac rhythm changes in varied resting and active states, and how food, gas and fluids move through the intestines.
Encapsulated in soft silicone, each device measures 40 millimeters long, 20 millimeters wide and 8 millimeters thick. Within that small footprint, the device contains a flash memory drive, tiny battery, electronic components, Bluetooth capabilities and two tiny microphones — one facing inward toward the body and another facing outward toward the exterior. By capturing sounds in both directions, an algorithm can separate external (ambient or neighboring organ) sounds and internal body sounds.
“Lungs don't produce enough sound for a normal person to hear,” Bharat said. “They just aren’t loud enough, and hospitals can be noisy places. When there are people talking nearby or machines beeping, it can be incredibly difficult. An important aspect of our technology is that it can correct for those ambient sounds.”
Not only does capturing ambient noise enable noise canceling, it also provides contextual information about the patients’ surrounding environments, which is particularly important when treating premature babies.
“Irrespective of device location, the continuous recording of the sound environment provides objective data on the noise levels to which babies are exposed,” said Dr. Wissam Shalish, a neonatologist at the Montreal Children’s Hospital and co-first author of the paper. “It also offers immediate opportunities to address any sources of stressful or potentially compromising auditory stimuli.”
When developing the new devices, the researchers had two vulnerable communities in mind: premature babies in the neonatal intensive care unit (NICU) and post-surgery adults. In the third trimester during pregnancy, babies’ respiratory systems mature so babies can breathe outside the womb. Babies born either before or in the earliest stages of the third trimester, therefore, are more likely to develop lung issues and disordered breathing complications.
Particularly common in premature babies, apneas are a leading cause of prolonged hospitalization and potentially death. When apneas occur, infants either do not take a breath (due to immature breathing centers in the brain) or have an obstruction in their airway that restricts airflow. Some babies might even have a combination of the two. Yet, there are no current methods to continuously monitor airflow at the bedside and to accurately distinguish apnea subtypes, especially in these most vulnerable infants in the clinical NICU.
“Many of these babies are smaller than a stethoscope, so they are already technically challenging to monitor,” said Dr. Debra E. Weese-Mayer, a study co-author, chief of autonomic medicine at Ann & Robert H. Lurie Children’s Hospital of Chicago and the Beatrice Cummings Mayer Professor of Autonomic Medicine at Feinberg. “The beauty of these new acoustic devices is they can non-invasively monitor a baby continuously — during wakefulness and sleep — without disturbing them. These acoustic wearables provide the opportunity to safely and non-obtrusively determine each infant’s ‘signature’ pertinent to their air movement (in and out of airway and lungs), heart sounds and intestinal motility day and night, with attention to circadian rhythmicity. And these wearables simultaneously monitor ambient noise that might affect the internal acoustic ‘signature’ and/or introduce other stimuli that might affect healthy growth and development.”
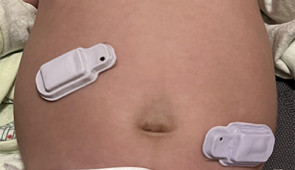
In collaborative studies conducted at the Montreal Children’s Hospital in Canada, health care workers placed the acoustic devices on babies just below the suprasternal notch at the base of the throat. Devices successfully detected the presence of airflow and chest movements and could estimate the degree of airflow obstruction with high reliability, therefore allowing identification and classification of all apnea subtypes.
“When placed on the suprasternal notch, the enhanced ability to detect and classify apneas could lead to more targeted and personalized care, improved outcomes and reduced length of hospitalization and costs,” Shalish said. “When placed on the right and left chest of critically ill babies, the real-time feedback transmitted whenever the air entry is diminished on one side relative to the other could promptly alert clinicians of a possible pathology necessitating immediate intervention.”
In children and infants, cardiorespiratory and gastrointestinal problems are major causes of death during the first five years of life. Gastrointestinal issues, in particular, are accompanied by reduced bowels sounds, which could be used as an early warning sign of digestion issues, intestinal dysmotility and potential obstructions. So, as part of the pilot study in the NICU, the researchers used the devices to monitor these sounds.
In the study, premature babies wore sensors at four locations across their abdomen. Early results aligned with measurements of adult intestinal motility using wire-based systems, which is the current standard of care.
“When placed on the abdomen, the automatic detection of reduced bowel sounds could alert the clinician of an impending (sometimes life-threatening) gastrointestinal complication,” Shalish said. “While improved bowel sounds could indicate signs of bowel recovery, especially after a gastrointestinal surgery.”
“Intestinal motility has its own acoustic patterns and tonal qualities,” Weese-Mayer said. “Once an individual patient's acoustic ‘signature’ is characterized, deviations from that personalized signature have potential to alert the individual and health care team to impending ill health, while there is still time for intervention to restore health.”
In addition to offering continuous monitoring, the devices also untethered NICU babies from the variety of sensors, wires and cables connected to bedside monitors.
Accompanying the NICU study, researchers tested the devices on adult patients, which included 35 adults with chronic lung diseases and 20 healthy controls. In all subjects, the devices captured the distribution of lung sounds and body motions at various locations simultaneously, enabling researchers to analyze a single breath across a range of regions throughout the lungs.
“As physicians, we often don’t understand how a specific region of the lungs is functioning,” Bharat said. “With these wireless sensors, we can capture different regions of the lungs and assess their specific performance and each region’s performance relative to one another.”
In 2020, cardiovascular and respiratory diseases claimed nearly 800,000 lives in the U.S., making them the first and third leading causes of death in adults, according to the Centers for Disease Control and Prevention. With the goal of helping guide clinical decisions and improve outcomes, the researchers hope their new devices can slash these numbers to save lives.
“Lungs can make all sorts of sounds, including crackling, wheezing, rippling and howling,” Bharat said. “It’s a fascinating microenvironment. By continuously monitoring these sounds in real time, we can determine if lung health is getting better or worse and evaluate how well a patient is responding to a particular medication or treatment. Then we can personalize treatments to individual patients.”
The study, “Wireless broadband acousto-mechanical sensors as body area networks for continuous physiological monitoring,” was supported by the Querrey-Simpson Institute for Bioelectronics at Northwestern University. The paper’s co-first authors are Jae-Young Yoo of Northwestern, Seyong Oh of Hanyang University in Korea and Wissam Shalish of the McGill University Health Centre.
Editor's note: Northwestern University has financial interests (equities, royalties) in Sibel Health, Inc., a joint owner of related Intellectual Property. Rogers has a financial interest in and affiliation with Sibel Health, Inc.
<iframe width="560" height="315" src="https://www.youtube.com/embed/beBBkGuO3QU" frameborder="0" allow="accelerometer; autoplay; encrypted-media; gyroscope; picture-in-picture" allowfullscreen></iframe>
For photo credits, please see caption for each image




Co-corresponding author
Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering and Neurological Surgery

