Nanomaterial fabric destroys nerve agents in battlefield-relevant conditions
Metal-organic framework-based composites don’t need liquid water to work
- Link to: Northwestern Now Story
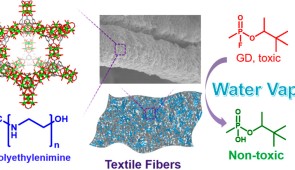
Northwestern University scientists have successfully combined a nanomaterial effective at destroying toxic nerve agents with textile fibers. This new composite material one day could be integrated into protective suits and face masks for use by people facing hazardous conditions, such as chemical warfare.
The material, a zirconium-based metal-organic framework (MOF), degrades in minutes some of the most toxic chemical agents known to mankind: VX and soman (GD), a more toxic relative of sarin.
“With the correct chemistry, we can render toxic gases nontoxic,” said Omar K. Farha, associate professor of chemistry in the Weinberg College of Arts and Sciences, who led the research. “The action takes place at the nanolevel.”
The study was published recently in the Journal of the American Chemical Society.
The authors write that their work represents, to the best of their knowledge, the first example of the use of MOF composites for the efficient catalytic hydrolysis of nerve agent simulants without using liquid water and toxic volatile bases -- a major advantage.
The new composite material integrates MOFs and non-volatile polymeric bases onto textile fibers. The researchers found the MOF-coated textiles efficiently detoxify nerve agents under battlefield-relevant conditions using the gaseous water in the air. They also found the material stands up over a long period of time to degrading conditions, such as sweat, atmospheric carbon dioxide and pollutants.
These features bring the promising material closer to practical use in the field.
“MOFs can capture, store and destroy a lot of the nasty material, making them very attractive for defense-related applications,” said Farha, a member of the International Institute for Nanotechnology.
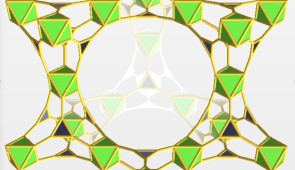
MOFs are well-ordered, lattice-like crystals. The nodes of the lattices are metals, and organic molecules connect the nodes. Within their very roomy pores, MOFs can effectively capture gases and vapors, such as nerve agents.
It is these roomy pores that also can pull enough water from the humidity in the air to drive the chemical reaction in which water is used to break down the bonds of the nerve agent.
The approach developed at Northwestern seeks to replace the technology currently in use: activated carbon and metal-oxide blends, which are slower to react to nerve agents. Because the MOFs are built from simple components, the new approach is scalable and economical.
The research was supported by the Defense Threat Reduction Agency (grants HDTRA1-18-1-0003 and CB3934) and the National Science Foundation Graduate Research Fellowship (grant DGE-1842165).
The title of the paper is “Integration of Metal–Organic Frameworks on Protective Layers for Destruction of Nerve Agents under Relevant Conditions.” The first authors are Zhijie Chen and Kaikai Ma, postdoctoral fellows in Farha’s research group.
(Source contact: Omar Farha at o-farha@northwestern.edu)
Multimedia Downloads
Assets