Scientists at Northwestern University have developed a new approach that directly combats the progression of neurodegenerative diseases like Alzheimer’s disease and amyotrophic lateral sclerosis (ALS).
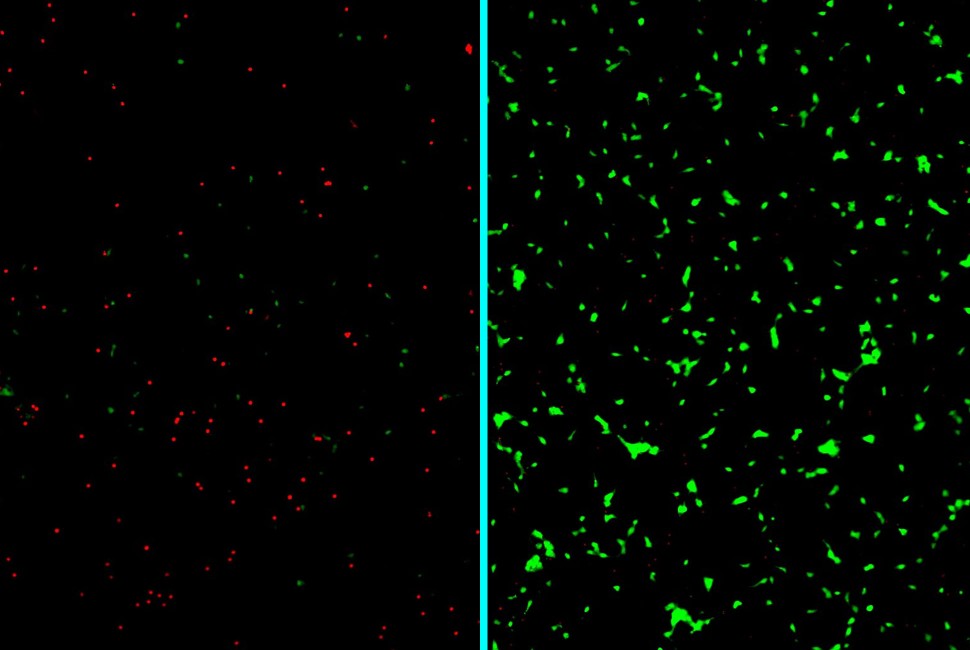
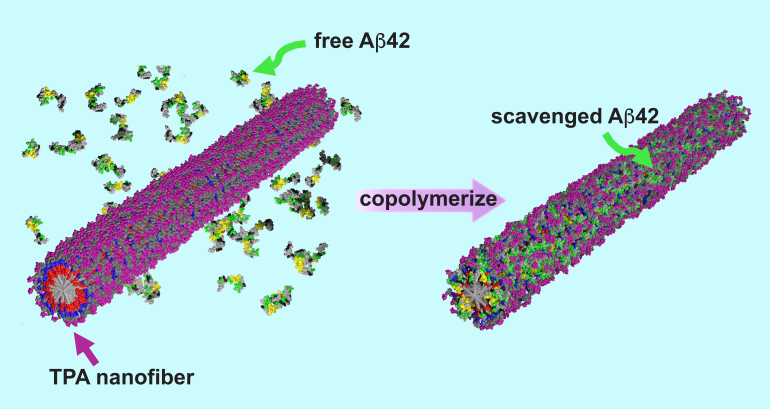
In these devastating illnesses, proteins misfold and clump together around brain cells, which ultimately leads to cell death. The innovative new treatment effectively traps the proteins before they can aggregate into the toxic structures capable of penetrating neurons. The trapped proteins then harmlessly degrade in the body.
The “clean-up” strategy significantly boosted the survival of lab-grown human neurons under stress from disease-causing proteins.
Selected as an ACS Editors’ Choice article, the study was published today (May 14) in the Journal of the American Chemical Society.
“Our study highlights the exciting potential of molecularly engineered nanomaterials to address the root causes of neurodegenerative diseases,” said Northwestern’s Samuel I. Stupp, the study’s senior author. “In many of these diseases, proteins lose their functional folded structure and aggregate to make destructive fibers that enter neurons and are highly toxic to them.
“By trapping the misfolded proteins, our treatment inhibits the formation of those fibers at an early stage. Early stage, short amyloid fibers, which penetrate neurons, are believed to be the most toxic structures. With further work, we think this could significantly delay progression of the disease.”
A pioneer in regenerative medicine, Stupp is the Board of Trustees Professor of Materials Science and Engineering, Chemistry, Medicine and Biomedical Engineering at Northwestern, where he has appointments in the McCormick School of Engineering, Weinberg College of Arts and Sciences and Feinberg School of Medicine. He also is the founding director of the Center for Regenerative Nanomedicine (CRN). Zijun Gao, a Ph.D. candidate in Stupp’s laboratory, is the paper’s first author.
The Stupp group led the development and characterization of the new therapeutic materials. Co-corresponding author Zaida Alvarez — a researcher at the Institute for Bioengineering of Catalonia (IBEC) in Spain, former postdoctoral fellow in Stupp's laboratory and current visiting scholar at CRN — led testing of the therapies in human neurons.
A sugar-coated solution
According to the World Health Organization, as many as 50 million people worldwide might have a neurodegenerative disorder. Most of these diseases are characterized by the accumulation of misfolded proteins in the brain, leading to the progressive loss of neurons. While current treatments offer limited relief, a dire need for new therapies remains.
The molecules in this novel therapeutic concept break down into harmless lipids, amino acids and sugars. That means there are fewer adverse side effects.”
regenerative nanomedicine pioneer
“The advantage of peptide-based drugs is that they degrade into nutrients,” Stupp said. “The molecules in this novel therapeutic concept break down into harmless lipids, amino acids and sugars. That means there are fewer adverse side effects.”
Over the years, Stupp’s research group has designed many peptide-based materials for different therapeutic purposes. To develop a peptide amphiphile to treat neurodegenerative diseases, his team added an extra ingredient: a natural sugar called trehalose.
“Trehalose is naturally occurring in plants, fungi and insects,” Gao said. “It protects them from changing temperatures, especially dehydration and freezing. Others have discovered trehalose can protect many biological macromolecules, including proteins. So, we wanted to see if we could use it to stabilize misfolded proteins.”
Instability is key
When added to water, the peptide amphiphiles self-assembled into nanofibers coated with trehalose. Surprisingly, the trehalose destabilized the nanofibers. Although it seems counterintuitive, this decreased stability exhibited a beneficial effect.