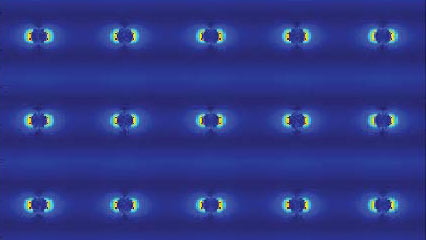
Researchers have developed a tiny nanolaser that can function inside of living tissues without harming them.
Just 50 to 150 nanometers thick, the laser is about 1/1,000th the thickness of a single human hair. At this size, the laser can fit and function inside living tissues, with the potential to sense disease biomarkers or perhaps treat deep-brain neurological disorders, such as epilepsy.
Developed by researchers at Northwestern and Columbia Universities, the nanolaser shows specific promise for imaging in living tissues. Not only is it made mostly of glass, which is intrinsically biocompatible, the laser can also be excited with longer wavelengths of light and emit at shorter wavelengths.

“Longer wavelengths of light are needed for bioimaging because they can penetrate farther into tissues than visible wavelength photons,” said Northwestern’s Teri Odom, who co-led the research. “But shorter wavelengths of light are often desirable at those same deep areas. We have designed an optically clean system that can effectively deliver visible laser light at penetration depths accessible to longer wavelengths.”
The nanolaser also can operate in extremely confined spaces, including quantum circuits and microprocessors for ultra-fast and low-power electronics.
The paper was published today (Sept. 23) in the journal Nature Materials. Odom co-led the work with P. James Schuck at Columbia University’s School of Engineering.
While many applications require increasingly small lasers, researchers continually run into the same roadblock: Nanolasers tend to be much less efficient than their macroscopic counterparts. And these lasers typically need shorter wavelengths, such as ultraviolet light, to power them.
“This is bad because the unconventional environments in which people want to use small lasers are highly susceptible to damage from UV light and the excess heat generated by inefficient operation,” said Schuck, an associate professor of mechanical engineering.
Odom, Schuck and their teams were able to achieve a nanolaser platform that solves these issues by using photon upconversion. In upconversion, low-energy photons are absorbed and converted into one photon with higher energy. In this project, the team started with low-energy, “bio-friendly” infrared photons and upconverted them to visible laser beams. The resulting laser can function under low powers and is vertically much smaller than the wavelength of light.
Excitingly, our tiny lasers operate at powers that are orders of magnitude smaller than observed in any existing lasers.”
mechanical engineer
“Our nanolaser is transparent but can generate visible photons when optically pumped with light our eyes cannot see,” said Odom, the Charles E. and Emma H. Morrison Professor of Chemistry in Northwestern’s Weinberg College of Arts and Sciences. “The continuous wave, low-power characteristics will open numerous new applications, especially in biological imaging.”
“Excitingly, our tiny lasers operate at powers that are orders of magnitude smaller than observed in any existing lasers,” Schuck said.
The study, “Ultralow-threshold, continuous-wave upconverting lasing from subwavelength plasmons,” was supported by the National Science Foundation (award number DMR-1608258), the Vannevar Bush Faculty Fellowship from the U.S. Department of Defense (award number N00014-17-1-3023) and the U.S. Department of Energy (DE-AC02-05CH11231). Angel Fernandez-Bravo of Berkeley Lab's Molecular Foundry and Northwestern's Danqing Wang are the paper’s co-first authors.
Odom is a member of Northwestern’s International Institute of Nanotechnology, Chemistry of Life Processes Institute and the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.