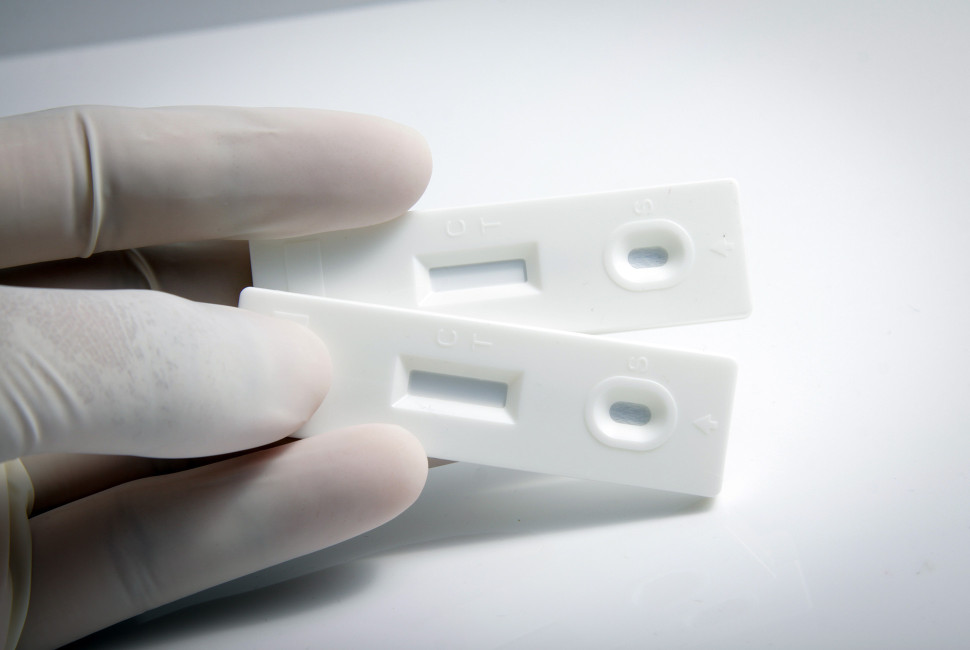
Scientists at Northwestern University have developed the fastest test yet for diagnosing hepatitis C virus (HCV). The highly accurate diagnostic delivers results to patients in just 15 minutes — up to 75% faster than other rapid HCV tests. This speed is crucial for kickstarting patients’ treatment before they leave their appointment, potentially preventing painful, expensive complications and even death.
The background
HCV can lead to a chronic hepatitis C infection, which affects an estimated 50 million people globally and causes approximately 242,000 deaths annually, largely due to resulting cirrhosis and liver cancer. While the infection is curable with an 8- to 12-week course of medication, treatment rates remain low partially due to lack of affordable and easily accessible diagnostic tests.
Hepatitis C diagnosis is a two-step process. First, an antibody test determines if the patient was exposed to the virus. If the result is positive, a second PCR test detects the presence of viral RNA to determine if the patient has an active infection. Health care workers typically send the PCR sample to a central lab for testing, which can take days or even weeks. Then, the patient must return to their doctor to receive results. Although the Food and Drug Administration has approved one other point-of-care HCV test, results still take 40 to 60 minutes — much longer than the typical clinical appointment.
What’s new
“We were able to develop a diagnostic test that can be performed at the point of care during a patient’s clinical visit, which could enable same-day diagnosis and treatment in support of HCV elimination efforts,” said corresponding author Sally McFall, who developed the test. McFall is co-director of the Center for Innovation in Global Health Technologies (CIGHT) at Northwestern University McCormick School of Engineering and the Robert J. Havey, MD Institute for Global Health.
The test has demonstrated excellent analytical and clinical performance, McFall said. It also could play a critical role in the World Health Organization’s ambitious goal to eliminate HCV by 2030.
How they did it
The research, published in The Journal of Infectious Diseases, is a collaboration between engineering and infectious disease faculty at Northwestern. To develop the new rapid polymerase chain reaction (PCR) test for HCV, the scientists used the DASH® (Diagnostic Analyzer for Specific Hybridization) PCR platform, which was originally developed at Northwestern University to detect COVID from samples collected with nasal swabs. The HCV test uses a whole blood specimen demonstrating the flexibility of the DASH platform.
The scientists shipped DASH® analyzers and DASH® HCV cartridges to collaborators at Johns Hopkins University, who then used 97 clinical specimens to evaluate the test’s performance. Their independent analysis confirmed 100% agreement when compared to commercial platforms.
What’s next
“This test could revolutionize HCV care in the U.S. and globally by dramatically improving diagnosis, accelerating treatment uptake and enabling more people to be cured faster,” said study co-author Dr. Claudia Hawkins, director of the Havey Institute for Global Health’s Center for Global Communicable and Emerging Infectious Diseases at Northwestern. “By reducing delays and simplifying testing pathways, it has the potential to save millions of lives from the devastating liver-related complications of untreated HCV.”
Notes
The study was supported by the National Cancer Institute, the National Institute of Biomedical Imaging and Bioengineering and the National Institute of Allergy and Infectious Disease, all of the National Institutes of Health. Sally McFall was one of the inventors of the DASH technology and owns stock in Nuclein, LLC, who is commercializing the platform.