Clean hydrogen’s iridium problem? Solved in an afternoon
New megalibrary accelerates discovery of iridium alternative for hydrogen production
- Link to: Northwestern Now Story
EMBARGOED UNTIL 8 A.M. EDT (U.S.) ON TUESDAY, AUGUST 19, 2025
- New material rivals iridium for water splitting reaction at a fraction of the cost
- Discovered using megalibraries, a high-speed materials synthesis and discovery tool, along with artificial intelligence and machine learning
- Findings demonstrate a faster, AI-ready approach to discover advanced, optimized materials
EVANSTON, Ill. --- For decades, researchers around the world have searched for alternatives to iridium, an extremely rare, incredibly expensive metal used in the production of clean hydrogen fuels.
Now, a powerful new tool has found one — within a single afternoon.
Invented and developed at Northwestern University, that tool is called a megalibrary. The world’s first nanomaterial “data factory,” each megalibrary contains millions of uniquely designed nanoparticles on one tiny chip. In collaboration with researchers from the Toyota Research Institute (TRI), the team used this technology to discover commercially relevant catalysts for hydrogen production. Then, they scaled up the material and demonstrated it could work within a device — all in record time.
With a megalibrary, scientists rapidly screened vast combinations of four abundant, inexpensive metals — each known for its catalytic performance — to find a new material with performance comparable to iridium. The team discovered a wholly new material that, in laboratory experiments, matched or in some cases even exceeded the performance of commercial iridium-based materials, but at a fraction of the cost.
This discovery doesn’t just make affordable green hydrogen a possibility; it also proves the effectiveness of the new megalibrary approach, which could completely change how researchers find new materials for any number of applications.
The study was published today (Aug. 19) in the Journal of the American Chemical Society (JACS).
“We’ve unleashed arguably the world’s most powerful synthesis tool, which allows one to search the enormous number of combinations available to chemists and materials scientists to find materials that matter,” said Northwestern’s Chad A. Mirkin, the study’s senior author and primary inventor of the megalibrary platform. “In this particular project, we have channeled that capability toward a major problem facing the energy sector. That is: How do we find a material that is as good as iridium but is more plentiful, more available and a lot cheaper? This new tool enabled us to find a promising alternative and to find it rapidly.”
A nanotechnology pioneer, Mirkin is the George B. Rathmann Professor of Chemistry at Northwestern’s Weinberg College of Arts and Sciences; professor of chemical and biological engineering, biomedical engineering and materials science and engineering at the McCormick School of Engineering; and executive director of the International Institute for Nanotechnology. Mirkin co-led the work with Ted Sargent, the Lynn Hopton Davis and Greg Davis Professor of Chemistry at Weinberg, professor of electrical and computer engineering at McCormick and executive director of the Paula M. Trienens Institute for Sustainability and Energy.
‘Not enough iridium in the world’
As the world moves away from fossil fuels and toward decarbonization, affordable green hydrogen has emerged as a critical piece of the puzzle. To produce clean hydrogen energy, scientists have turned to water splitting, a process that uses electricity to split water molecules into their two constituent components — hydrogen and oxygen.
The oxygen part of this reaction, called the oxygen evolution reaction (OER), however, is difficult and inefficient. OER is most effective when scientists use iridium-based catalysts, which have significant disadvantages. Iridium is rare, expensive and often obtained as a byproduct from platinum mining. More valuable than gold, iridium costs nearly $5,000 per ounce.
“There’s not enough iridium in the world to meet all of our projected needs,” Sargent said. “As we think about splitting water to generate alternative forms of energy, there’s not enough iridium from a purely supply standpoint.”
‘Full army deployed on a chip’
Mirkin, who introduced the megalibraries in 2016, decided with Sargent that finding new candidates to replace iridium was a perfect application for his revolutionary tool. While materials discovery is traditionally a slow and daunting task filled with trial and error, megalibraries enable scientists to pinpoint optimal compositions at breakneck speeds.
Each megalibrary is created with arrays of hundreds of thousands of tiny, pyramid-shaped tips to print individual “dots” onto a surface. Each dot contains an intentionally designed mix of metal salts. When heated, the metal salts are reduced to form single nanoparticles, each with a precise composition and size.
“You can think of each tip as a tiny person in a tiny lab,” Mirkin said. “Instead of having one tiny person make one structure at a time, you have millions of people. So, you basically have a full army of researchers deployed on a chip.”
And the winner is…
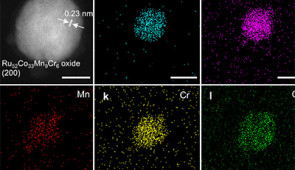
In the new study, the chip contained 156 million particles, each made from different combinations of ruthenium, cobalt, manganese and chromium. A robotic scanner then assessed how well the most promising particles could perform an OER. Based on these tests, Mirkin and his team selected the best-performing candidates to undergo further testing in the laboratory.
Eventually, one composition stood out: a precise combination of all four metals (Ru52Co33Mn9Cr6 oxide). Multi-metal catalysts are known to elicit synergistic effects that can make them more active than single-metal catalysts.
“Our catalyst actually has a little higher activity than iridium and excellent stability,” Mirkin said. “That’s rare because oftentimes ruthenium is less stable. But the other elements in the composition stabilize ruthenium.”
The ability to screen particles for their ultimate performance is a major new innovation. “For the first time, we were not only able to rapidly screen catalysts, but we saw the best ones performing well in a scaled-up setting,” said Joseph Montoya, a senior staff research scientist at TRI and study co-author.
In long-term tests, the new catalyst operated for more than 1,000 hours with high efficiency and excellent stability in a harsh acidic environment. It is also dramatically cheaper than iridium — about one-sixteenth of the cost.
“There’s lots of work to do to make this commercially viable, but it’s very exciting that we can identify promising catalysts so quickly — not only at the lab scale but for devices,” Montoya said.
Just the beginning
By generating massive high-quality materials datasets, the megalibrary approach also lays the groundwork for using artificial intelligence (AI) and machine learning to design the next generation of new materials. Northwestern, TRI and Mattiq, a Northwestern spinout company, have already developed machine learning algorithms to sift through the megalibraries at record speeds.
Mirkin says this is only the beginning. With AI, the approach could scale beyond catalysts to revolutionize materials discovery for virtually any technology, such as batteries, biomedical devices and advanced optical components.
“We’re going to look for all sorts of materials for batteries, fusion and more,” he said. “The world does not use the best materials for its needs. People found the best materials at a certain point in time, given the tools available to them. The problem is that we now have a huge infrastructure built around those materials, and we’re stuck with them. We want to turn that upside down. It’s time to truly find the best materials for every need — without compromise.”
About the study
The study, “Accelerating the pace of oxygen evolution reaction catalyst discovery through megalibraries,” was supported by the Toyota Research Institute, Mattiq and the Army Research Office, a directorate of the U.S. Army Combat Capabilities Development Command Army Research Laboratory (award number W911NF-23-1-0285). This publication was made possible with the support of The Bioindustrial Manufacturing and Design Ecosystem (BioMADE); the content expressed herein is that of the authors and does not necessarily reflect the views of BioMADE.
This material is based on research sponsored by the Air Force under agreement number FA8650-21-2-5028. The U.S. Government is authorized to reproduce and distribute reprints for governmental purposes notwithstanding any copyright notation thereon.
The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the Air Force or the U.S. Government.
Chad Mirkin is a founder of, and has a financial relationship with Mattiq. Ted Sargent is on the advisory board of Mattiq and has an equity holding.
Multimedia Downloads
Images from the study
Interview the Experts