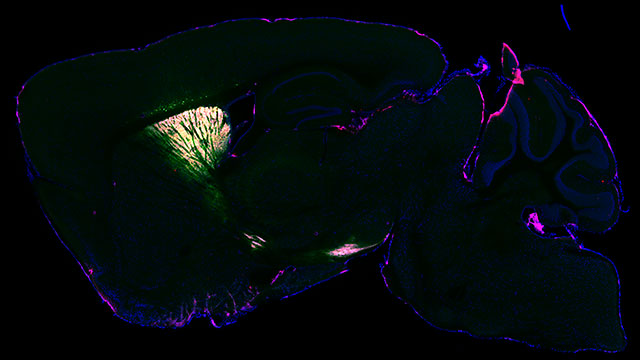
For the first time, researchers have developed a successful approach for identifying proteins inside different types of neurons in the brain of a living animal.
Led by Northwestern University and the University of Pittsburgh, the new study offers a giant step toward understanding the brain’s millions of distinct proteins. As the building blocks of all cells including neurons, proteins hold the keys to better understanding complex brain diseases such as Parkinson’s and Alzheimer’s, which can lead to the development of new treatments.
The study was published today (Aug. 11) in the journal Nature Communications.
In the new study, researchers designed a virus to send an enzyme to a precise location in the brain of a living mouse. Derived from soybeans, the enzyme genetically tags its neighboring proteins in a predetermined location. After validating the technique by imaging the brain with fluorescence and electron microscopy, the researchers found their technique took a snapshot of the entire set of proteins (or proteome) inside living neurons, which can then be analyzed postmortem with mass spectroscopy.

“Similar work has been done before in cellular cultures. But cells in a dish do not work the same way they do in a brain, and they don’t have the same proteins in the same places doing the same things,” said Northwestern’s Yevgenia Kozorovitskiy, senior author of the study. “It’s a lot more challenging to do this work in the complex tissue of a mouse brain. Now we can take that proteomics prowess and put it into more realistic neural circuits with excellent genetic traction.”
By chemically tagging proteins and their neighbors, researchers can now see how proteins work within a specific, controlled area and how they work with one another in a proteome. Along with the virus carrying the soybean enzyme, the researchers also used their virus to carry a separate green fluorescent protein.
“The virus essentially acts as a message that we deliver,” Kozorovitskiy said. “In this case, the message carried this special soybean enzyme. Then, in a separate message, we sent the green fluorescent protein to show us which neurons were tagged. If the neurons are green, then we know the soybean enzyme was expressed in those neurons.”
Kozorovitskiy is the Soretta and Henry Shapiro Research Professor of Molecular Biology, an associate professor of neurobiology in Northwestern’s Weinberg College of Arts and Sciencesand a member of the Chemistry of Life Processes Institute. She co-led the work with Matthew MacDonald, an assistant professor of psychiatry at the University of Pittsburgh Medical Center.
Protein targeting plays catch-up
While genetic targeting has completely transformed biology and neuroscience, protein targeting has woefully lagged behind. Researchers can amplify and sequence genes and RNA to identify their exact building blocks. Proteins, however, cannot be amplified and sequenced in the same manner. Instead, researchers have to divide proteins into peptides and then put them back together, which is a slow and imperfect process.
Proteins are the ultimate effectors in our cells. Understanding where proteins are, how they work and how they work relative to each other is really important.”
neurobiologist
“We have been able to gain a lot of traction with genetic and RNA sequencing, but proteins have been out of the loop,” Kozorovitskiy said. “Yet everyone recognizes the importance of proteins. Proteins are the ultimate effectors in our cells. Understanding where proteins are, how they work and how they work relative to each other is really important.”
“Mass spectroscopy-based proteomics is a powerful technique,” said Vasin Dumrongprechachan, a Ph.D. candidate in Kozorovitskiy’s laboratory and the paper’s first author. “With our approach, we can start mapping the proteome of various brain circuits with high precision and specificity. We can even quantify them to see how many proteins are present in different parts of neurons and the brain.”
Next step: Better understanding brain diseases
Now that this new system has been validated and is ready to go, the researchers can apply it to mouse models for disease to better understand neurological illnesses.
“We are hoping to extend this approach to start identifying the biochemical modifications on neuronal proteins that occur during specific patterns of brain activity or with changes induced by neuroactive drugs to facilitate clinical advances,” Dumrongprechachan said.
“We look forward to taking this to models related to brain diseases and connect those studies to postmortem proteomics work in the human brain,” Kozorovitskiy said. “It’s ready to be applied to those models, and we can’t wait to get started.”
The study, “Cell-type and subcellular compartment-specific APEX2 proximity labeling reveals activity-dependent nuclear proteome dynamics in the striatum,” was supported by the National Institute of Mental Health (award numbers R56MH113923, R01MH117111 and R01MH118497), National Institute of Neurological Disorders and Stroke (award number R01NS107539), National Science Foundation (CAREER 1846234), the American Heart Association (award number 19PRE34380056), the Beckman Young Investigator Award, Searle Scholar Award, Rita Allen Foundation Scholar Award and Sloan Research Fellowship.