‘2.0’ of COVID-19 vaccine quickly kicks immune system into high gear
Breakthrough infections were better controlled in mice with new version of vaccine
- Link to: Northwestern Now Story
Let the record show, the current COVID-19 vaccines work, and they work well. But what is the next step in vaccine development, especially amid increasing rates of breakthrough infections and emergence of variants of concern?
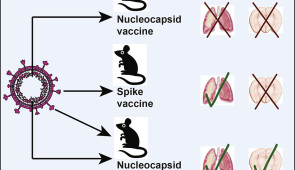
In a new Northwestern Medicine study in mice, researchers took one of the current vaccines, which is based on the novel coronavirus’ infamous spike protein, and added a different antigen, the nucleocapsid protein, to form a new, potentially improved version of the COVID vaccine. The nucleocapsid protein, which is an internal RNA-binding protein, may help kick the immune system into high gear much more quickly than the spike protein is capable of since it is among the most rapidly and highly expressed proteins in coronaviruses.
“At this point, we’re just trying to figure out ‘What should the 2.0 vaccines be?’” said senior and corresponding study author Pablo Penaloza-MacMaster, an assistant professor of microbiology-immunology at Northwestern University Feinberg School of Medicine. “It seems like adding nucleocapsid to the vaccine renders it more protective, relative to having only the spike.”
The combination vaccine improved protection against breakthrough infections in mice. It is the first study to compare side by side the efficacy against breakthrough infection of a current, spike-based vaccine with a vaccine that includes an additional antigen, in this case the nucleocapsid protein.
The study was published August 17 in the journal Cell Reports.
Curtailing a recent infection ‘from the get-go’
The well-known coronavirus spike protein is located outside the virus, whereas the nucleocapsid protein is present inside the virus. The nucleocapsid protein is one of the most rapidly and abundantly expressed proteins, making it the perfect target for early detection by the T cell response, Penaloza-MacMaster said. He believes this is what made it so effective at preventing breakthrough infections in mice.
“When a cell gets infected by SARS-CoV-2, you want the T cells to recognize this infected cell as soon as possible,” Penaloza-MacMaster said. “If your T cells can detect one of the early proteins expressed during the coronavirus life cycle, then you will be better able to extinguish the infection from the get-go, before the virus starts disseminating exponentially.”
Another advantage of incorporating nucleocapsid in next-generation COVID vaccines is that this protein is more conserved (or similar) among SARS-CoV-2 variants and even among other coronaviruses.
“We have to start thinking about a future vaccine that not only targets the spike, which is variable, but also the parts of the virus that don’t change that much, like the nucleocapsid.”
The ‘first side-by-side comparison’
The scientists immunized mice with vaccines made from just the SARS-CoV-2 spike protein, just the nucleocapsid protein or both proteins combined. After several weeks, the researchers exposed the mice via their noses with SARS-CoV-2 and measured viral loads in the mice’s respiratory systems (the proximal site of virus exposure) or their nervous system (the distant site of challenge) 72 hours after exposure to capture a breakthrough infection.
The studies allowed the scientists to measure how well each vaccine protected against breakthrough infection, and determine how the infection travels from the respiratory system to other sites of the body (nervous system).
The results could be the basis for developing more effective vaccines not only in terms of preventing, but also clearing breakthrough infections.”
Protecting against long-term neurological manifestation of COVID
Scientists are still collecting data to determine whether vaccinated individuals who have a breakthrough COVID infection develop long-term neurological symptoms. In this study, the scientists found evidence of viral infection in the brains of mice that were immunized with a current spike-based vaccine.
“I’m a little worried to see that the mice had viral loads in the brains because this suggests that perhaps with the current spike-based vaccines, these breakthrough infections could potentially get through to the brain,” Penaloza-MacMaster said. “We don’t know if that’s just in mice or whether it constitutes a general phenomenon. We’re investigating that right now, but a technical challenge is that, due to ethical reasons, we cannot sample brain tissue of vaccinated humans to evaluate breakthrough infection.”
He hopes the new combination vaccine may better protect the brain from developing neurological symptoms if a breakthrough infection occurs.
Tanushree Dangi, a microbiology and immunology postdoctoral fellow, and Nicole Palacio, a Ph.D. candidate in the Penaloza-MacMaster’s laboratory, are co-authors on the paper. This study was a collaboration with Justin Richner and Jacob Class, investigators at the University of Illinois Chicago.
Funding for the study was provided by the National Institutes of Health (grant DP2DA051912), and the Emerging and Re-Emerging Pathogens Program (EREPP) program at Northwestern University.