Most people simply take ibuprofen when they get a headache. But for someone with hydrocephalus – a potentially life-threatening condition in which excess fluid builds up in the brain -- a headache can indicate a serious problem that can result in a hospital visit, thousands of dollars in scans, radiation and sometimes surgery.
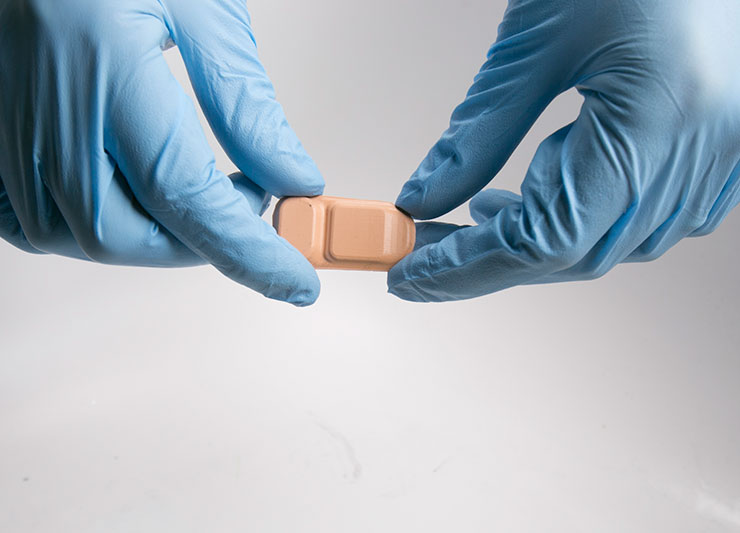
A new wireless, Band-Aid-like sensor developed at Northwestern University could revolutionize the way patients manage hydrocephalus and potentially save the U.S. health care system millions of dollars. The current standard of care involves the surgical implantation of a tube known as a ‘shunt’ to drain this excess fluid out of the brain. Shunts have a nearly 100 percent failure rate over 10 years, and diagnosing shunt failure is notoriously difficult.
A Northwestern Medicine clinical study successfully tested the device, known as a wearable shunt monitor, on five adult patients with hydrocephalus. The findings were published Oct. 31 in the journal Science Translational Medicine.
Device could change the lives of a million patients
A device like this would be life changing for Willie Meyer, 26, who has undergone 190 surgeries, spent virtually every holiday in the emergency room and almost missed his high school graduation because of emergency brain surgeries.
“I’m trying to live a normal life, and I really can’t because of the headaches,” Meyer said.
Hydrocephalus affects one in 1,000 live births and is about as common as Down Syndrome. Treating the condition costs an estimated $50,000 per patient per year, and costs the U.S. health care system about $1 billion per year. If left untreated, hydrocephalus can lead to death.
To treat the condition, surgeons implant a straw-like catheter, or shunt, into the brain to drain the excess fluid to another part of the body. More than 1 million Americans live with shunts and the constant threat of their failure.
When a shunt fails, the patient can experience headaches, nausea and low energy. A patient experiencing any of these symptoms must visit a hospital because if their symptoms are caused by a malfunctioning shunt, it could be life threatening. Once at the hospital, the patient must get a CT scan or an MRI and sometimes must undergo surgery to see if the shunt is working properly.
The groundbreaking new sensor, developed by the Rogers Research Group at Northwestern, allowed patients in the study to determine within five minutes of placing it on their skin if fluid was flowing through their shunt. The soft and flexible sensor uses measurements of temperature and heat transfer to non-invasively tell if and how much fluid is flowing through.
“We envision you could do this while you’re sitting in the waiting room waiting to see the doctor,” said co-lead author Siddharth Krishnan, a fifth-year Ph.D. student in the Rogers Research Group. “A nurse could come and place it on you and five minutes later, you have a measurement.”
‘A Band-Aid talking to a cellphone’
A very small rechargeable battery is built into the sensor. The device is Bluetooth enabled so it can talk to a smartphone and deliver the readings via an Android app.
“At the end of the day, from a patient perspective, it looks like a Band-Aid that’s talking to their cellphone,” said co-senior author John A. Rogers.
“It’s a wearable device with a specific but useful mode of operation that’s addressing an unmet need in clinical medicine,” said Rogers, the Louis Simpson and Kimberly Querrey Professor of Materials Science and Engineering, Biomedical Engineering and Neurological Surgery in the McCormick School of Engineering and a professor of neurological surgery at Northwestern University Feinberg School of Medicine. “There’s nothing like this out there today.”
Next steps: Clinical pediatric trial and moving beyond the lab
A larger pediatric clinical trial will be starting soon at Ann & Robert H. Lurie Children’s Hospital of Chicago with the goal of targeting this very vulnerable population. The study authors are also working on outsourced production on the scale of a few hundred sensors to support this study and further develop the technology.
“We realized this couldn’t be one of those research projects that stopped with the paper,” Krishnan said. “The last few months have been about taking this lab-scale prototype that we’ve essentially built by hand and thinking about how to make that something that can be manufactured externally with the same level of performance.”
That would be a relief for Willie Meyer’s mother, Beth Meyer. She said she learned Willie had hydrocephalus when he was just six weeks old. His initial shunt functioned properly for his first two and a half years until he complained to Beth that “his hair hurt.” Willie was too young to know how to vocalize his headache, which is common among pediatric hydrocephalus patients and is a source of stress for their caregivers.
“Shunt malfunction symptoms, like headaches or sleepiness, are things kids can have for lots of reasons, like the flu,” said co-senior author Dr. Matthew Potts, assistant professor of neurological surgery at Feinberg and a Northwestern Medicine physician. “So if a child has these symptoms, it’s very hard to know, and every time your kid says they have a headache or feels a little sleepy, you automatically think, ‘Is this the shunt?’ We believe that this device can spare patients a lot of the danger and costs of this process.”
When Willie was younger, Beth said they needed to bring along copies of his CT scans when they traveled, and would research local neurosurgeons in the area in case Willie got a headache.
“The biggest part of hydrocephalus is the unpredictability and not being able to know from one day to the next whether you’ll be in the emergency room or at home,” Beth said. “This type of device would get rid of the uncertainty.”
Co-lead author Dr. Amit Ayer, a sixth-year neurosurgery resident at Northwestern Medicine and an MBA student at Kellogg School of Management at Northwestern, has treated Willie’s hydrocephalus for the last four years. Ayer said his patients are a driving force behind his motivation to get the device to market.
“Our patients want to know when they can actually use the device and be part of the trial,” Ayer said. “I want to get it out there, so we can help make their lives better.”
Ayer and Rogers are co-founders of Rhaeos, Inc., a company that is commercializing the technology.
Hydrocephalus can affect adults and children. Often the child is born with the condition, whereas in adults, it can be acquired from some trauma-related injury, such as bleeding inside the brain or a brain tumor, Potts said.
Given the uncertainty and failure rates associated with shunts, the technology could create immense savings and improve the quality of life for nearly a million people in the U.S. alone.
The science behind the device
The sensor advances concepts in skin-like "epidermal electronics," which the Rogers Research Group has been working on for nearly a decade.
The sensor uses a thermal transport measurement, which means the sensor uses tiny amounts of thermal power to minimally increase the temperature of the skin. If the shunt is working and the excess cerebral spinal fluid is draining properly, the sensor will measure a characteristic heat signature. Similarly, if there is no flow because the shunt has malfunctioned, the sensor will be able to quickly indicate that through heat flow measurements.
The team tested the device in the laboratory before heading to the clinic to perform a pilot study on five patients at Northwestern Memorial Hospital. The team could detect clear differences in cases between measurements over working shunts and on adjacent confusing control locations with no flow.
“This means if someone wants to check if their shunt is working, say, when they have a headache, they can quickly do what we call a ‘spot measurement,’” said co-lead author Tyler Ray, a postdoctoral research fellow in the Rogers Research Group. “This device can also measure flow throughout the day enabling, for the first time, the possibility of continuously monitoring shunt performance. This can lead to important insights into the dynamics of cerebral spinal fluid flow previously inaccessible with current diagnostic tools and flow measurement techniques.”
This study was funded by the Center for Bio-Integrated Electronics at Northwestern with small additional funding through the Dixon Translational Grant at Feinberg.
Reporters should contact the SciPak (MedPak) team at 202-326-6440 or scipak@aaas.org to receive an official version of the paper.