Those who have tried to pry a mussel from wood or rock know how stubborn the underwater mollusk is. Its gluey secret has long captivated scientists. For years, researchers have attempted to replicate the extraordinary adhesive and its properties in the lab, targeting some of the eight proteins mussels secrete and use to attach to surfaces.
Now, using a novel method to arrange molecules, researchers at Northwestern University have created a material that performs even better than the glue they were trying to mimic. Their findings expand on how these protein-like polymers can be used as a platform to create new materials and therapeutics.
“The polymer could be used as an adhesive in a biomedical context, which means now you could stick it to a specific tissue in the body and keep other molecules nearby in one place, which would be useful in wound healing or repair,” said Northwestern’s Nathan Gianneschi.
Gianneschi, who led the study, is the Jacob and Rosaline Cohn Professor of Chemistry in the Weinberg College of Arts and Sciences.
Proteins like those secreted by mussel feet exist around nature. Appearing at times stretchy, strong and sticky, the protein frameworks show up in insect wings and legs, spider silk and mussel feet. Scientists know the exact primary sequences of amino acids that make up many such proteins, yet have trouble replicating the complicated natural process while still maintaining the extraordinary qualities.
The paper’s first author Or Berger, a postdoctoral researcher in Gianneschi’s lab who studies peptides — these very chains of amino acids — came with an idea for how to arrange amino acid building blocks differently to replicate the properties rather than directly copy the structure of mussel proteins.
As associate director of the International Institute for Nanotechnology, Gianneschi has built much of his lab around the idea of mimicking proteins in function by using polymer chemistry. Within precision therapeutics, drug therapies like antibodies and other small molecules combat some diseases, where a nanocarrier is used to deliver a drug to a target more effectively. But Gianneschi says replicating proteins could approach biological problems differently, by changing interactions inside and between cells that are involved in the progression of disease, or between cells, tissues and materials.
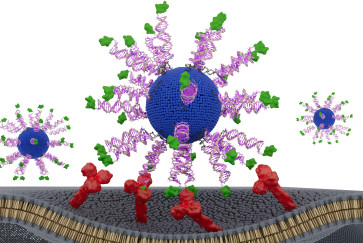
“Proteins arrange amino acids as chains, but instead we took them and arranged them in parallel, on a dense synthetic polymer backbone,” Gianneschi said. “The same platform technology that will be used for future therapeutics has really become potentially interesting in materials science.”
The result was something that looks like a brush of peptides rather than looping together amino acids in a straight line as a chain. While the novel process may seem like adding an additional step, forming protein-like polymers (PLPs) skips several steps, requiring researchers to form peptides in a readily available synthesizer and insert them into the tightly packed backbone rather than going through tedious steps of protein expression.
To test the new material’s efficacy, the researchers applied either the polymer material or the native mussel protein to glass plates. The researchers placed cells on the plates and then, after washing them, assessed how many cells were present, either attached or not, to gauge how well the materials performed. They found the PLP formed a cellular superglue, leaving the most cells attached compared to the native mixture and untreated plate.
“We only meant to mimic the mussel’s properties, but when we went and tested several times, we actually got better properties than the native material in these settings.”
The team hopes the model can be widely applicable across other proteins that repeat their sequence. They hypothesize such a platform could perform better than their native counterparts because they are denser and scalable. Gianneschi said this is the first of many papers to discuss polymer-based protein mimics, and he is already thinking about applications for future materials.
Resilin, for example, a stretchy protein found in insect legs and wings, could be used to make flexible drones and other robotics.
Gianneschi and Berger are inventors on pending intellectual property in this space. Gianneschi also is a professor of biomedical engineering and materials science and engineering in the McCormick School of Engineering and a member of the Chemistry of Life Processes Institute, Simpson Querrey Institute and Robert H. Lurie Comprehensive Cancer Center of Northwestern University. He is the co-founder of a company, Grove Biopharma, that seeks to develop versions of these materials as translational therapeutics.
The study was led by an all-Northwestern team with help from the labs of chemical and biological engineering professors Muzhou Wang and Danielle Tullman-Ercek.
The Jacob and Rosaline Cohn Professorship is supported in part by alumni Patrick G. ’59, ’09 H and Shirley W. Ryan ’61, ’19 H through the Ryan Family Chair Challenge, which matched gifts made by other Northwestern supporters to establish new endowed professorships across a wide range of disciplines from screen and stage writing to nanotechnology and biomedical engineering. The chairs will provide a dedicated source of funds for the scholarly activities of chair holders like Gianneschi, underwriting salaries for these faculty and members of their research teams.