Northwestern University researchers have developed the first physics-based metric to predict whether or not a person might someday suffer an aortic aneurysm, a deadly condition that often causes no symptoms until it ruptures.
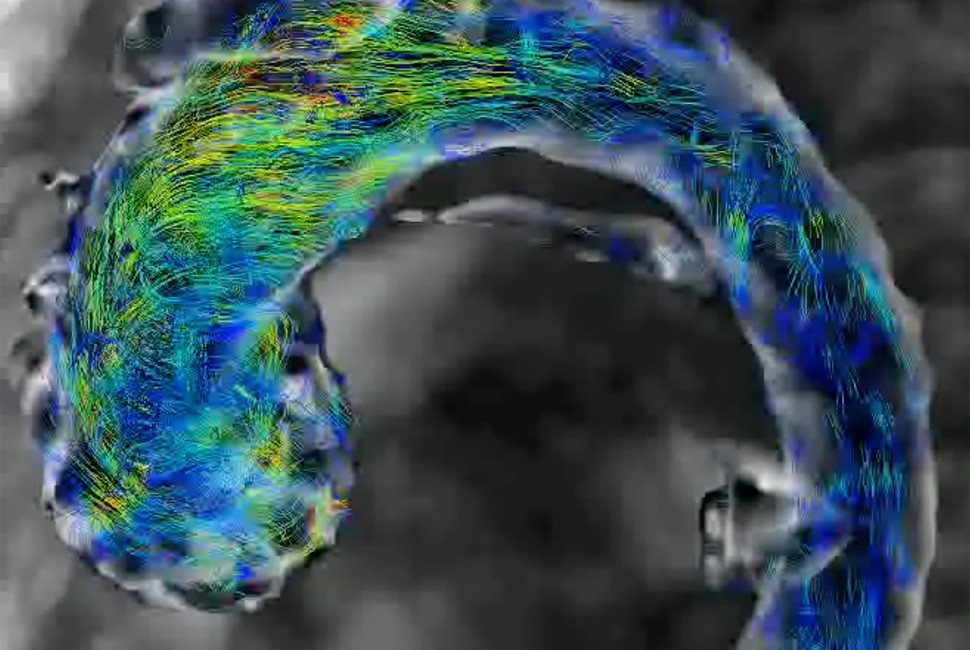
In the new study, the researchers forecasted abnormal aortic growth by measuring subtle “fluttering” in a patient’s blood vessel. As blood flows through the aorta, it can cause the vessel wall to flutter, similar to how a banner ripples in the breeze. While stable flow predicts normal, natural growth, unstable flutter is highly predictive of future abnormal growth and potential rupture, the researchers found.
Called the “flutter instability parameter” (FIP), the new metric predicted future aneurysm with 98% accuracy on average three years after the FIP was first measured. To calculate a personalized FIP, patients only need a single 4D flow magnetic resonance imaging (MRI) scan.
Using the clinically measurable, predictive metric, physicians could prescribe medications to high-risk patients to intervene and potentially prevent the aorta from swelling to a dangerous size.
Fluttering is a mechanical signature of future growth.”
The research was published in the journal Nature Biomedical Engineering.
“Aortic aneurysms are colloquially referred to as ‘silent killers’ because they often go undetected until catastrophic dissection or rupture occurs,” said Northwestern’s Neelesh A. Patankar, senior author of the study. “The fundamental physics driving aneurysms has been unknown. As a result, there is no clinically approved protocol to predict them. Now, we have demonstrated the efficacy of a physics-based metric that helps predict future growth. This could be transformational in predicting cardiac pathologies.”
An expert on fluid dynamics, Patankar is a professor of mechanical engineering at Northwestern’s McCormick School of Engineering. He co-led the study with Tom Zhao, who specializes in first principles biomechanics.
Growing danger
An aortic aneurysm occurs when the aorta (the largest artery in the human body) swells to greater than 1.5 times its original size. As it grows, the aorta’s wall weakens. Eventually, the wall becomes so weak that it can no longer withstand the pressure of blood flowing through it, causing the aorta to rupture. Although rare, an aortic rupture is usually unpredictable and almost always fatal.
Several prominent people have died from aortic aneurysm, including Grant Wahl, a sports journalist who died suddenly one year ago at the 2022 FIFA World Cup. Other celebrity deaths include John Ritter, Lucille Ball and Albert Einstein.
“Most people don’t realize they have an aneurysm unless it is accidentally detected when they receive a scan for an unrelated issue,” Patankar said. “If physicians detect it, they can suggest lifestyle changes or prescribe medication to lower blood pressure, heart rate and cholesterol. If it goes undetected, it can rupture, which is an immediate catastrophic event.”
“If it ruptures when the person is outside of a hospital, the death rate is close to 100%,” Zhao added. “The blood supply to the body stops, so critical organs like the brain can no longer function.”
Removing the guesswork
For current standard of care, physicians estimate chance of rupture based on risk factors (such as age or smoking history) and the size of the aorta. To monitor a growing aorta, physicians track it with regular imaging scans. If the aorta starts to grow too quickly or become too large, then a patient often will undergo a surgical graft to reinforce the vessel wall, an invasive procedure that carries its own risks.
“Our collective lack of understanding makes it hard to monitor aneurysm progression,” Zhao said. “Doctors need to regularly track the size of an aneurysm by imaging its location every one to five years depending on how fasts it grew previously and whether the patient has any associated diseases. Over this ‘wait and see’ period, an aneurysm can fatally burst.”
To remove the guesswork from predicting future aneurysms, Patankar, Zhao and their collaborators sought to capture the fundamental physics underlying the problem. In extensive mathematical work and analyses, they discovered that problems arise when the fluttering vessel wall transitions from stable to unstable. This instability either causes or signals an aneurysm.
“Fluttering is a mechanical signature of future growth,” Patankar said.
Capturing the underlying physics
To quantify the transition from stability to instability, the researchers combined blood pressure, aorta size, stiffness of the aortic wall, shear stress on the wall and pulse rate. The resulting number (or FIP) characterizes the exact interaction between blood pressure and wall stiffness that ultimately triggers fluttering instability.
“Physicians have known that these factors — blood pressure, heartbeat frequency and aortic size — were involved, but they didn’t know how to quantify it,” Patankar said. “It turns out the combination of the factors is what’s important. A patient might have an unstable wall but a normal-sized aorta, so their doctor would not even realize there was a problem.”
Surprisingly, the researchers discovered that instability tends to arise when the wall is more flexible. This finding directly contradicts common knowledge that aortic stiffness is a sign of disease.
“We show that the less stiff it is, then the more at-risk the patient is for future growth and rupture,” Zhao said. “This is because once the aorta reaches a certain size, the body tries to stiffen it up to seemingly protect it from future growth. But the ones that are still growing are less stiff. The aorta will flutter if the wall is more compliant.”
Validating the metric
To test the new metric, the researchers reviewed 4D flow MRI data from 117 patients who underwent cardiac imaging to monitor heart disease and from 100 healthy volunteers. Based on this MRI, the researchers assigned each patient a personalized FIP. In this metric, zero marks the threshold between stable and unstable.
For patients with an FIP below zero, their aorta was unlikely to experience abnormal growth. Researchers predicted that patients with an FIP higher than zero, however, would experience abnormal growth and future rupture.
"In establishing prognostic value of this quantitative metric for cardiovascular 4D flow MRI, we can significantly improve the value of imaging offered as standard of care to patients with aneurysms," said Dr. Ethan Johnson, the study’s co-first author and a postdoctoral fellow in cardiovascular imaging at Northwestern University Feinberg School of Medicine.
When the researchers compared these predictions to follow-up MRIs or physician diagnoses, they discovered their predictions were accurate in 98% of the cases. Although the FIP predicted future growth on average three years after the initial MRI (when the FIP was calculated), the researchers say this metric may even offer a more granular view of heart health on a daily or monthly basis.
“The period of one to eight years is the time range in which our clinical data sits,” Zhao said. “Not the total time interval in which the FIP is necessarily effective.”
Next, Patankar, Zhao and their team plan to explore if the FIP can provide clues into how other heart conditions develop. They also are studying if patient-specific FIP can indicate which prevention methods are most effective in stopping aneurysm progression.