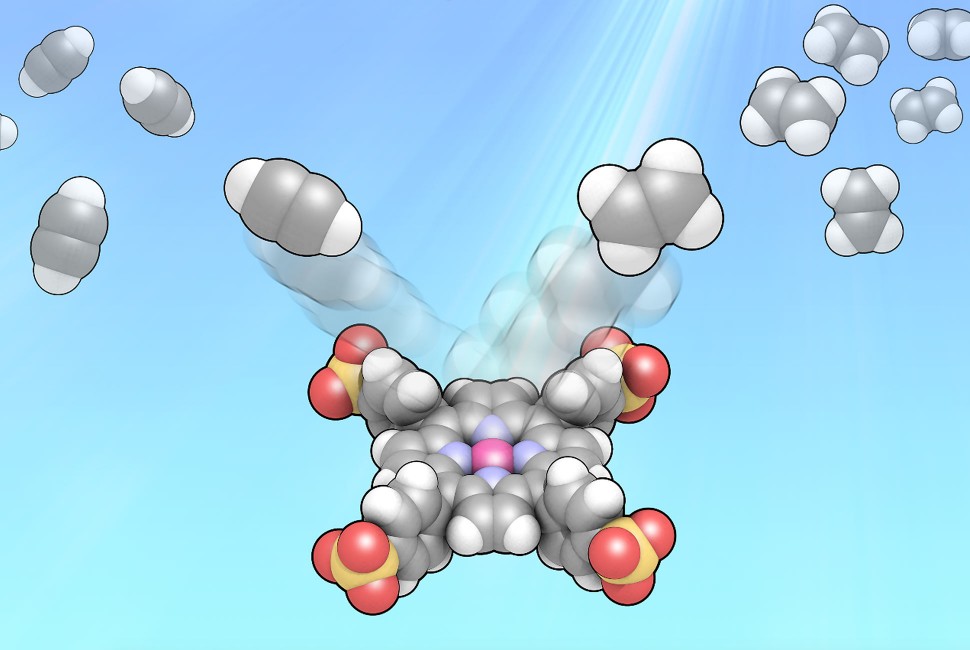
Northwestern University chemists have taken inspiration from plants to revolutionize the way an important industrial chemical is made.
In a first for the field, the Northwestern team used light and water to convert acetylene into ethylene, a widely used, highly valuable chemical that is a key ingredient in plastics.
While this conversion typically requires high temperatures and pressures, flammable hydrogen and expensive metals to drive the reaction, Northwestern’s photosynthesis-like process is much less expensive and less energy intensive. Not only is the new process environmentally friendly, it also works incredibly well — successfully converting nearly 100% of acetylene into ethylene.
“In industry, this method requires energy-intensive processes that need high temperatures, an external feed of flammable hydrogen gas and materials containing noble metals, which are expensive and difficult to obtain,” said Northwestern’s Francesca Arcudi, co-first author of the study. “Our new strategy solves all these issues at once. It operates using light and water in place of high temperatures and hydrogen. And instead of expensive metals, we use naturally abundant, inexpensive materials.”
The resulting strategy worked shockingly well. Whereas the current industrial process results in 90% selectivity for ethylene, the Northwestern approach has 99% selectivity for ethylene.
“This is important because it’s a commodity chemical with high economic value,” said Northwestern’s Luka Ðorđević, co-first author of the study. “The more you can produce without waste, the better.”
The study was published in the journal Nature Chemistry. It is the first report of researchers using light to convert acetylene to ethylene.