There is plenty of scientific evidence that the health of a mother can impact the health of her child. Now a Northwestern University study flips that relationship around: Researchers have discovered the health of the fertilized embryo determines the functional health of the mother, which has implications for healthy aging, stress resilience and suppression of protein damage.
Essentially, a bad egg does good by protecting the mother from cellular stress, ensuring she lives longer and is healthy enough to produce the next generation.
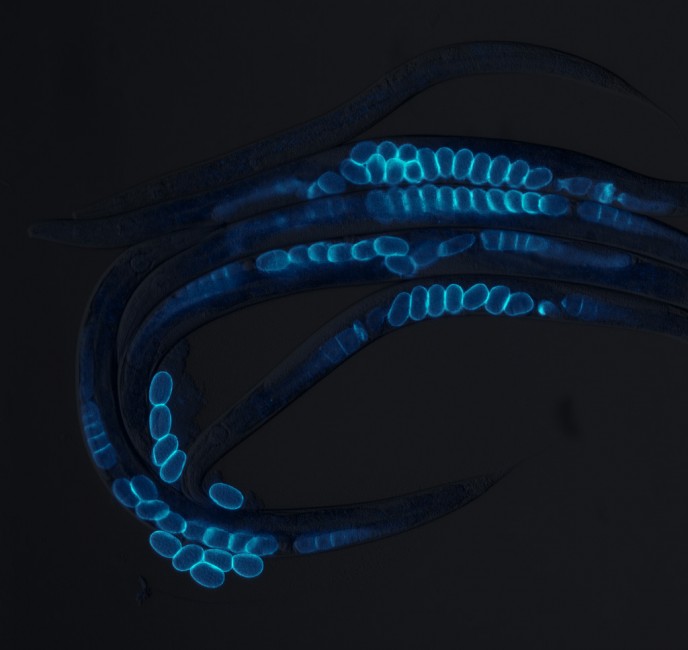
Led by molecular biologist Richard I. Morimoto and postdoctoral fellow Ambre J. Sala, the research team studied maternal health span using a popular research tool, the transparent roundworm C. elegans. This animal, whose cellular properties and protective mechanisms are similar to that of humans, is used by scientists to better understand aspects of human biology, such as aging and neurodegenerative disease.
Using the power of a genetic screen, the researchers discovered that if the eggshell of a fertilized egg is damaged, a molecular signal is sent to the mother that protects her from the negative effects of a human protein associated with neurodegeneration. They found that this signal also protects the mother from environmental stress, allowing her to better survive adverse conditions. This gives her a longer functional health span so she has more time to produce healthy eggs.
“The success and future of any species is about the quality of its progeny,” Morimoto said. “Now we know that progeny quality ensures maternal health.”
Morimoto is an expert on how organisms sense and respond to physiologic and environmental stress on the molecular and cellular level in biology, aging and neurodegenerative disease. He is the Bill and Gayle Cook Professor of Molecular Biosciences and director of the Rice Institute for Biomedical Research in Northwestern’s Weinberg College of Arts and Sciences.
This communication between child and parent is about protein quality control. Specifically, the researchers found that when the eggshell’s vitelline layer is damaged, that’s when the fertilized egg sends a signal that restores stress resilience and protein homeostasis, or proteostasis, in the mother.
The vitelline layer, found in all metazoans that use eggs, including humans, is the extracellular coat that surrounds and protects the developing embryo. Proteostasis is the processes by which cells maintain protein health, keeping important proteins folded and functional, for good overall health.
The study was published online recently by the journal Genes and Development. It also will appear in the May 2020 print issue of the journal. Morimoto is the corresponding author, and Sala is the first author.
These findings build on an earlier study by the Morimoto lab that looked at the regulation of proteostasis by an animal’s reproductive system. In that 2015 study, the researchers found that adult cells in C. elegans abruptly begin their downhill slide when an animal reaches reproductive maturity. After the animal starts to reproduce, germline stem cells throw a genetic switch that starts the aging process by turning off protective cell stress responses that protect against molecular damage as occurs in Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and other diseases of protein conformation.
In this new study, Morimoto’s team shows that communication between the embryo and mother in reproductive adults also regulates proteostasis, stress resilience and the mother’s health span. An implication of these results is that unhealthy progeny promotes the health span of the mother by preventing the occurrence of protein damage associated with proteins that cause neurodegeneration in humans.
“Aging is about a lack of protein quality control,” Morimoto said. “We found if the eggshell is damaged, the mother survives longer and has time to have good eggs and healthy offspring.”
The research was supported by grants from the National Institutes of Health (National Institute on Aging grants R56AG059579, R37AG026647, RF1AG057296 and P01AG054407) and the Daniel F. and Ada L. Rice Foundation.
The title of the paper is “Embryo Integrity Regulates Maternal Proteostasis and Stress Resilience.”