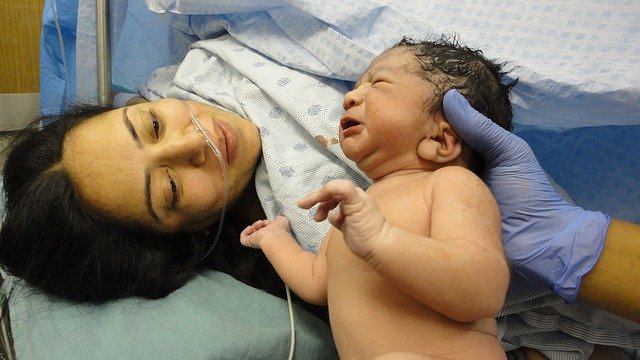
Whether it’s used to detect skin cancer or diagnose diabetes, artificial intelligence (AI) is becoming a go-to solution in health care.
Now, thanks to a team of researchers from Northwestern University Feinberg School of Medicine and Pennsylvania State University, AI machine learning might one day determine the health of a woman’s placenta after childbirth for a fraction of the cost and time.
Placentas can provide critical information about long-term health outcomes for a mother and her baby. Yet in the U.S., only 20% of placentas are assessed by pathology exams after delivery, in part because the cost, time and expertise required to analyze placentas are prohibitive.
The percentage of placentas assessed by pathology exams drops dramatically in lower-to-middle income countries around the world, where pathologists are either unavailable or not trained to assess placentas.
The research team’s patent-pending technology uses AI to analyze an image of each side of the placenta after delivery and produce a report with critical information that could impact the clinical care of the mother and child, such as whether the fetus received enough oxygen in the womb or if there is a risk of infection or bleeding.
This research could allow all placentas to be examined, reduce the number of normal placentas sent for full pathological examination and create a less resource-intensive path to analysis for research — all of which may positively benefit health outcomes for mothers and babies.
“The placenta drives everything to do with the pregnancy for the mom and baby, but we’re missing placental data on 95% of births globally,” said Alison Gernand, assistant professor of nutritional sciences in Penn State’s College of Health and Human Development.
Dr. Jeffrey Goldstein, assistant professor of pathology at Feinberg and a Northwestern Medicine pathologist, was a co-author on the study. He was responsible for interpreting the pathology images in the study and helping the computer learn how to analyze placenta images.
“Currently you have two options: Either no placental exam or a full exam, which takes three to five days or longer,” Goldstein said. “Ideally, we’d be analyzing every single placenta after childbirth. With this AI solution, if the AI is doing its job, it would be the first step to a full exam and provide preliminary information to make our jobs a little easier.”
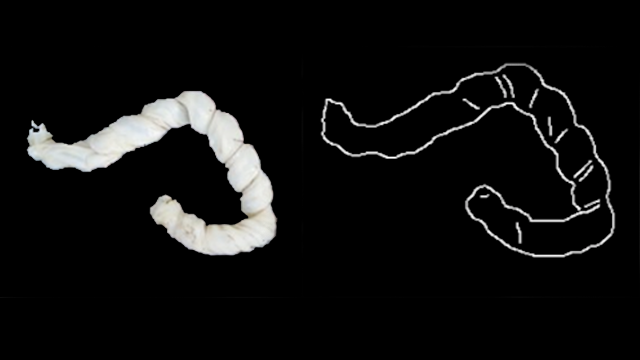
There are currently no evidence-based standards to determine when a placenta should be examined, and low-income countries and areas where home births are more prevalent often lack resources to conduct even a baseline placental analysis. This digital tool could offer a solution, as an individual would need only a smartphone or tablet with the appropriate software.
The team’s study was presented at the International Federation of Placenta Associations meeting held in Buenos Aires, Argentina, in September and at the International Conference on Medical Image Computing and Computer Assisted Intervention held in Shenzen, China, in October.
Fewer resources, quicker turnaround
The AI process would require fewer resources, allowing physicians to gather more comprehensive data to examine how placentas are linked to maternal and fetal health outcomes, and help examine placentas without special equipment — in minutes rather than days.
This AI solution would speed up this process dramatically, Goldstein said.
“Our goal is for a medical professional or trained birth attendant to take a photo, which, after analysis through licensed software, could provide immediate information that aids in the care of the mother and baby,” Gernand said.
For example, an umbilical cord with an abnormal insertion point or excessive twisting can be a predictor of neonatal stroke. Examination after a stillbirth could give a family information about whether future stillbirths may reoccur and help medical professionals advise them on possible interventions.
13,000 placental images, one AI solution
To create the system, the researchers analyzed 13,000 high-quality images of placentas and their corresponding pathology reports from Northwestern Memorial Hospital. Then, the researchers labeled a training set of images with data points critical to understanding the placenta, such as areas of incompleteness and the umbilical cord insertion point.
“Past analyses have typically examined features independently and used a limited number of images,” said James Wang, professor in Penn State’s College of Information Sciences and Technology. “Our tool leverages artificial intelligence and a large and comprehensive dataset to make multiple decisions at the same time by treating the different parts of the placenta as complementary. To our knowledge, this is the first system for comprehensive, automated placental analysis.”
The images were used to train neural networks using CPU and GPU servers that could automatically analyze new placental images to detect features linked to abnormalities and potential health risks. Their system produced predictions on unlabeled images efficiently, and comparisons with the original pathology reports demonstrated the system’s high accuracy and clinical potential.
Goldstein likens the machine-learning process to potty training a toddler.
“When an adult uses the bathroom, we don’t think twice about the process,” Goldstein said. “But when you’re teaching a toddler how to use the bathroom, you go through each step: Pull down your pants, underwear, sit on the toilet, etc. You really feel every single one of those steps. That’s how we teach a computer to learn, so it can learn to do these steps on its own.”
This research was supported by the Bill & Melinda Gates Foundation.